The modern regulatory landscape is continuously evolving—both at the state and federal level. Professionals who work in regulatory affairs or clinical research are likely already aware of the benefits of working in behind the scenes medical jobs like this one. However, if you're considering advancing your career to a regulatory affairs role without prior experience, it's important to understand the requirements of working in this industry.
Clinical research and regulatory affairs professionals require a comprehensive skill set, as well as a thorough educational background. Here's an overview of the skills you'll need to obtain in clinical research and regulatory affairs, how to develop these skills, and how you can jumpstart your career.
30 Clinical Research and Regulatory Affairs Skills
Working in clinical research and regulatory affairs requires an extensive list of skills. This includes hard skills (job specific proficiencies) and soft skills (interpersonal competencies that can easily transfer from one industry to another).
Hard Skills
According to our analysis of job postings data, the top hard skills required for clinical research and regulatory affairs professionals include:
- Pharmaceuticals
- Clinical trials
- Regulatory affairs
- Project management
- Clinical research
- Auditing
- Regulatory compliance
- Drug development
- Pre-clinical development
- Biology

Obtaining the necessary hard skills for a career in clinical research or regulatory affairs isn’t always easy. It typically requires prior working experience or advanced education in a clinical research or regulatory affairs program. If you're hoping to acquire these skills, it's important to find opportunities to obtain hands-on experience with each.
For example, if you're considering pursuing advanced education, make sure your program includes internship or co-op opportunities. These experiences not only provide valuable real-world exposure, but also offer networking opportunities to enhance your career prospects.
Soft Skills
As you progress toward more management-related roles in your career, soft skills become increasingly important. Effective leadership and the ability to keep a team motivated are crucial when overseeing a team.
According to our analysis of job postings data, the top soft skills required for these professionals include:
- Communications
- Research
- Management
- Leadership
- Writing
- Operations
- Detail oriented
- Problem solving
- Planning
- Interpersonal communications

The good news is that these skills can be developed in any position or role. This means that if you're interested in changing careers to a regulatory affairs or clinical research role, you don't have to wait until you have industry experience or additional education to demonstrate these soft competencies.
However, finding a program that emphasizes management skills as well as job-specific responsibilities can go a long way in fostering your soft and hard skills.
Software Skills
In addition to the hard and interpersonal skills required for regulatory affairs and clinical research professionals, it's also important to familiarize yourself with the software skills needed to advance your career in this field.
According to our analysis of job postings data, the top software skills required for these professionals include:
- Microsoft Office
- Microsoft Excel
- Microsoft PowerPoint
- Microsoft Outlook
- R (Programming language)
- Microsoft Word
- Python (Programming language)
- Microsoft Access
- Electronic Data Capture (EDC)
- Microsoft SharePoint

It's important to obtain programming skills in this industry because software is becoming a huge part of the medical industry. Safety risk management of software is an important task in healthcare, and often requires understanding the programming languages the software is built with.
Regulatory affairs and clinical research professionals also need to make sure they stay updated on technological trends such as electronic submission standards, artificial intelligence (AI) applications, and data standards. Proficiency in programming languages allows these professionals to stay updated with these technological advancements, aiding in the implementation and management of advanced systems and AI-driven tools within their regulatory practices.
Developing Your Regulatory Affairs and Clinical Research Skills
If you're hoping to develop your skill set in regulatory affairs, it's important to know how to go about doing so. The best way to get started in a new field like regulatory affairs and clinical research is to gain as much hands-on experience as possible. Internships, co-ops, and educational opportunities can help you sharpen your knowledge and hone specific skills.
Here's a more in-depth overview of how to prioritize and develop your regulatory affairs skill set.
Prioritize the Skills You Should Put on Your Resumé
While ideally you'll obtain every skill required for your profession, these skills often take time to develop. For this reason, it's important to know which skills to prioritize and highlight on your resumé.
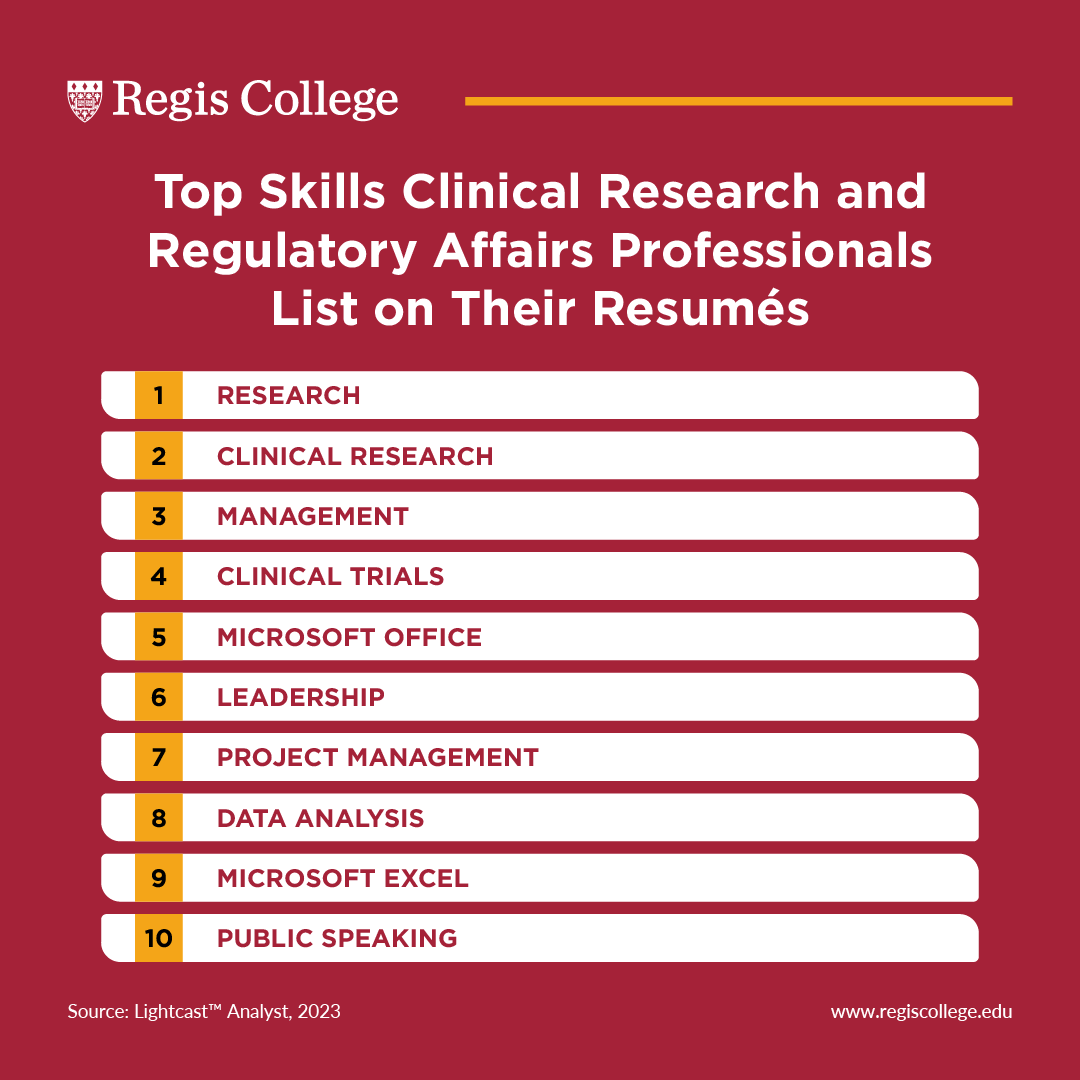
A good way to determine this is by considering the skills that others in a similar role list in their professional profiles. According to our analysis of job postings data, the top skills listed on regulatory affairs and clinical research professionals profiles include:
- Research
- Clinical research
- Management
- Clinical trials
- Microsoft Office
- Leadership
- Project management
- Data Analysis
- Microsoft Excel
- Public speaking
In order to stand out, it's important to ensure you have the same or similar competencies as others in your field. Otherwise, you might find yourself falling behind.
Advance Your Education
It's important not just to improve your skills, but to do so in a demonstrable way. One way to accomplish this is by advancing your education.
In both regulatory affairs and clinical research positions, advancing your education increases the scope of jobs you're able to apply for. Even if master's-level education can be substituted with experience, gaining additional education can help you stand out in your field and set you apart from other job candidates.
An excellent option for regulatory affairs professionals to advance their education is Regis College's Master of Science in Regulatory Affairs and Clinical Research Management (RCRM).
This program will help you develop your skills in:
- Regulatory affairs: Regulatory affairs competencies such as pharmaceuticals and regulatory compliance.
- Quality compliance: For products to pass regulatory standards, they must comply with quality standards to evaluate the quality of new products or processes.
- Clinical research management: The management focus of this degree will help you obtain valuable interpersonal skills as well, helping you become a better leader overall.
In addition, through this program you'll have the opportunity to network with regulatory affairs and clinical research professionals in Boston, which is a hub for the healthcare and life sciences industries. You'll obtain real-world experience through valuable internship opportunities, and receive support from Regis in finding a job post-graduation in your desired field.
Ready to Gain the Tools Needed to Advance Your Career?
If you're ready to skill-up and advance your regulatory affairs or clinical research career, consider applying to Regis College's RCRM program. You'll have the opportunity to specialize in either clinical research management or regulatory affairs, allowing you to focus on the niche you want to pursue.
Furthermore, you'll be given the chance to work with faculty who are experts in this subject, and obtain familiarity with a wide range of applications of regulatory affairs and clinical research in the life sciences industry.




